What Are Acids and Bases?
Lemons and Protons
When most of us hear the word acid, things such as lemons, batteries, and stomach juices come to mind. But what does it really mean to be an acid? In this guide, we'll dive into how chemists have defined acids (and bases) in the past and how those definitions got us to our current level of understanding, which goes way beyond lemons.
Before 1884, the definition of an acid had not advanced much beyond "the stuff in lemons that tastes sour." Thankfully, a chemistry hero named Svante Arrhenius came along and proposed a much more useful definition of an acid. The Arrhenius definition stated that acids are molecules that release a proton (H+) when they are in water. This definition can be expressed as a chemical equation:
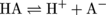
In this chemical equation, the molecule HA is the general formula for an acid. "A" is just a placeholder—it could be a picture of a banana for all we care. An example of an acid written in shorthand is HCl (hydrochloric acid), where a chlorine atom gets plopped where the "A" (or the banana) used to be.
HCl is a major component of stomach acid. When it's dissolved in water (or an aqueous environment), HCl breaks apart, or dissociates, fully into H+ and Cl- ions. The double-headed arrows in the equation above tell us that, like many chemical reactions, the dissociation of an acid is an equilibrium process. That means the reactants and products rapidly interconvert. For an acid like HCl, the equilibrium strongly favors the right-hand side of the equation as depicted by the larger top arrow in the equation above.
Even before Arrhenius' day, chemists and ordinary folks noticed that some substances could counteract the effects of acidic substances. In 1829, Sir James Murray supposedly mixed up some magnesium hydroxide to sooth the stomach ailments of some wealthy folks. This probably gave him major brownie points considering the diets of the aristocracy at that time probably consisted primarily of spicy hot salsa and hard alcohol. That's a heartburn commercial waiting to happen. The preparation became known as milk of magnesia, and was the first popularized stomach antacid.
Arrhenius knew that bases could neutralize acids, one such base being milk of magnesia. As he did for acids, Arrhenius proposed a general definition for bases as substances that release hydroxide (OH-) when they are in water. Again, chemical equations are our friends:

In this case, MOH (rhymes with Homer Simpson's "d'oh") is the general formula for an Arrhenius base where 'M' is usually a metal. Some examples are the bases NaOH, Mg(OH)2, and KOH.
Notice that an Arrhenius acid releases H+ and an Arrhenius base releases OH-. During a neutralization reaction, neutral H2O is magically produced from the H+ ions released from the acid and from the OH- ions released from the base (H+ + OH- = H2O). Therefore, Arrhenius's definitions could account for the mysterious reactions between basic and acidic substances, like magnesium hydroxide and stomach acid. Well done, Arrhenius.
Arrhenius Gets a Make Over
Eventually, other chemists realized that some substances seemed to be acidic or basic but didn't quite fit the Arrhenius definition. For example, ammonia (NH3) appeared to act as a base, but NH3 doesn't have any OH- molecules to release. What the what? Poor Arrhenius. He tried so hard to make a general definition, too.In 1923, two delightful chemists named Johannes Brønsted and Thomas Lowry proposed a new definition for acids and bases. Their definition could account for the behavior of squirrely molecules like NH3.

The Delightful Johannes Brønsted. (Image from here.)

The Slightly More Delightful Thomas Lowry. (Image from here.)
According to Brønsted and Lowry, an acid is a molecule that can transfer a proton (H+) to another molecule, and a base is a molecule that can accept a proton from another molecule. In short, a Brønsted-Lowry acid gives away protons and a Brønsted-Lowry base accepts protons. Any chemical reaction that involves a transfer of protons is an acid-base reaction.
One way to remember this is to think of a baseball pitcher as an acid. The pitcher transfers the baseball to home base just as an acid transfers a proton. Taking the baseball analogy further would mean that the catcher is analogous to a base since he accepts the baseball. The catcher sits at home base, too. That's one way to remember that a baseball catcher acts like a Brønsted-Lowry base. You're welcome.
Chemical equation time:
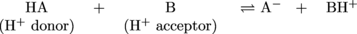
In this generic chemical equation, HA is acting as a Brønsted-Lowry acid by transferring its proton to molecule B. As a result, molecule B is acting as a Brønsted-Lowry base (or a catcher) by accepting the proton from HA.
There are two differences between this definition and the Arrhenius definition:
- First, the Brønsted-Lowry reaction does not necessarily have to take place in pure water. This is important for organic chemists who often need to predict the behaviors of acid-base reactions in other common solvents such as ethanol or benzene.
- In the Brønsted-Lowry equation, the proton is always transferred to another molecule. The proton does not end up all by its lonesome. Molecules are very social creatures and it turns out a lonesome proton probably does not exist in practice. Score another point for the Brønsted-Lowry acid-base definition.
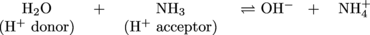
Presto. In this equation, when read left to right, NH3 is a Brønsted-Lowry base because it is accepting a proton from H2O. What if the equation was read right to left? After all, the double arrows mean that this reaction can go both ways.
By shifting our brains to the right hand side of the equation we see that the products are also acids and bases. The OH- is acting as a base because it accepts a proton to produce H2O. NH4+ is acting as an acid because it transfers a proton to produce NH3. The molecules on each side of the equation that differ by only a single proton are called conjugate acid-base pairs. In the following example, one pair is NH3 and NH4+ and the other pair is H2O and OH-:
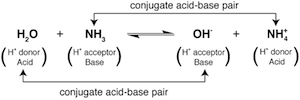
This chemical equation contains a little cozy acid-base family. Not to get all Barney on you. The relationship between the molecules differing by a single proton defines a conjugate acid-base pair. For a given conjugate acid-base pair, the molecule that is labeled the acid is said to have a conjugate base on the other side of the equation. Likewise, a molecule that is labeled a base has a conjugate acid on the other side of the equation.
For example, let's say you were the NH3 base in the equation above. You would describe the rest of your family like this: NH4+ is your conjugate acid, H2O is the acid you accept a proton from, and OH- is the conjugate base of the H2O acid. Alternatively, if you see yourself more as the OH- base, then H2O is your conjugate acid, NH4+ is the acid you accept a proton from, and NH3 is the conjugate base of the NH4+ acid.
Acid/Base Zombies vs. Acid/Base Humans
Now that we have a sense of how acids and bases are defined, we'll turn to acid and base strength. That is, how likely is it that a given acid will transfer its proton or that a given base will accept a proton? Check out the general chemical equation for a Brønsted-Lowry acid in water:
There is symmetry in this equation. Okay, maybe not quite as much symmetry as a snowflake, but it has symmetry nonetheless. Notice that there is an acid on both sides of the equation and a base on both sides of the equation. As a result, there is a competition between which acid gets to transfer its proton and which base gets to accept the proton. Proton fight! The outcome of this competition depends on just two factors:
- How strong a given acid or base is, and
- The concentrations of acid and base in the reaction relative to one another
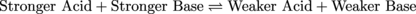
One of the toughest acids around is our old friend HCl. If we were to add some HCl to H2O the chemical equation describing the reaction would look like the following chemical equation. The equation below shows the reaction strongly favoring the right side (check out the big upper arrow pointing right):
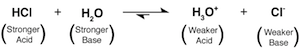
HCl approaches full dissociation when combined with H2O. This is because HCl is a much stronger acid than H3O+ and similarly, H2O is a much stronger base compared to Cl-. The proton therefore wants to be transferred from the stronger acid (HCl) to be accepted by the stronger base (H2O) resulting in the favored formation of H3O+ and Cl-.
The strength of an acid is relative. It depends on which other acid is in the ring fighting it out over the proton. By convention, acids are considered strong acids if they are much stronger than the acid H3O+, which is a pretty wimpy acid compared to HCl. A strong acid (like HCl) nearly completely dissociates when H2O acts as the proton acceptor.
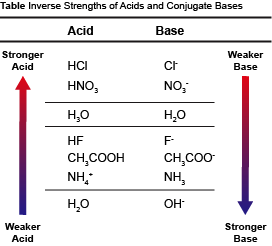
On the other hand, weak acids only partially dissociate in H2O. When a weak acid is put in the proverbial fighting ring with H2O, a significant portion of the protons just stay on the weak acid. Weak acids, by definition, have strong conjugate base partners. The strong conjugate base partner on the other side of the equation is just fine without a proton. The result is that a very small percentage of the weak acid protons get transferred. This inverse relationship between an acid and its conjugate base is illustrated in the table below.
Brain Snack
Check out the chemistry in your stomach and how bases are used to neutralize excess acids here.Louie Louie Oh No, We Gotta Go
Meet the new kids on the block (no, not these new kids). We're talking about Lewis Acid and Lewis Base.The "Lewis" concept of an acid or base—named after Gilbert Lewis—is even more general than the Brønsted-Lowry definition we talked about before. One thing to remember is that all Lewis acids are also Brønsted-Lowry acids and all Lewis bases are also Brønsted-Lowry bases. The Lewis definition is just another way of looking at the same overall property. It's kind of like saying there's more than one way to peel a banana. So how is the Lewis definition different?
Instead of focusing on the protons, Gilbert Lewis focused on electrons. He specifically considered lone-pair electrons. Here are Gilbert Lewis's definitions.
Lewis acid: An electron-pair acceptorIn the world of Lewis acids and Lewis bases, electrons are king.
Lewis base: An electron-pair donor
Let's look at typical Brønsted-Lowry base to see how it's also a Lewis base:
- NH3 has a lone pair of electrons on the nitrogen atom. (Remember the Lewis dot structure? These structures were also named after the chemistry hall-of-famer, Gilbert Lewis.)
- When a proton comes along, the lonely electrons plead with the proton to come join them.
- NH3 donates its pair of electrons to the proton.
- The proton is sucked up from solution. The electrons get to hang out with the proton and everyone is happy.
While all Brønsted-Lowry bases are also Lewis bases, not all Lewis acids are Brønsted-Lowry acids. (It's kind of like how all squares are rectangles, but not all rectangles are squares.) Al3+ is an example of a Lewis acid that is not a Brønsted-Lowry acid. After all, Al3+ has no protons to transfer as required by the Brønsted-Lowry definition. However, Al3+ accepts electron-pairs from water to form a coordinated Al(H2O)63+ complex. In this case, Al3+ is acting as a Lewis acid and H2O is acting as a Lewis base.
The Lewis concept of acids and bases can be very useful, particularly when thinking about molecules with acidic or basic properties but don't fit the Brønsted-Lowry definitions. However, for most chemists, the Brønsted-Lowry definition is good enough. From here on out we won't really talk about acids and bases in the 'Lewis' sense, but rather we'll use the good ole' Brønsted-Lowry definition.