Important Atomic Values
Atomic Number, Mass Number, and Isotopes, Oh My!
Chemistry can seem a lot like a foreign language. It's all about learning the lingo. Let's jump right in and get a little terminology out of the way.The sum of the number of protons plus the number of neutrons in an atom is called the mass number. The number of protons in a atom is called the atomic number. For example, the atomic number of helium is two, which means it has two protons. The mass number is four which means helium also has two neutrons. Remember: atomic number plus the number of neutrons equals the mass number. Don't worry. It's just algebra.
mass number = number of protons + number of neutrons
= atomic number + number of neutrons
For example, consider a particular boron atom with a mass number of 12 and an atomic number of 5. This means there are 5 protons and the number of neutrons is 12 – 5 = 7. All three quantities (atomic number, number of neutrons, and mass number) are positive whole numbers.
Just like in math class, there is special notation for passing along this information quickly. Check out the notation for helium below. Notice the helium atom is represented by the atomic symbol He. All elements have their own particular atomic symbol. To the left of the atomic symbol is the mass number (top) and the atomic number (bottom). You can find all of this snazzy information on the periodic table.

In most cases, atoms of a given element do not all have the same mass. Atoms that have the same atomic number but different mass numbers are called isotopes. In other words, isotopes are elements that have a differing number of neutrons. We knew there was more to being a neutron than just being neutral.
The most basic element, hydrogen (H), has three isotopes. The most common isotope is called hydrogen and has one proton and no neutrons. The deuterium isotope has one proton and one neutron, and tritium has one proton and two neutrons. Because neutrons are neutral in charge, hydrogen, deuterium, and tritium all have the same number of protons and the same number of electrons. Remember, neutrons have a significant amount of mass so tritium is heavier than deuterium which is heavier than hydrogen. Got it? Good!
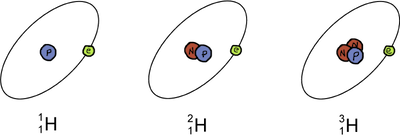
Isotopes of the element Hydrogen (H).
Check out the diagram above. Notice the changes in the symbols of the three hydrogen isotopes. The only difference is the mass number which is located at the top to the left of the elemental symbol. This is just another way to rephrase what we've already said: Isotopes are the same element with differing numbers of neutrons. Don't you love how chemistry makes sense sometimes?