Physics—Semester B
Ohm Sweet Ohm
- Credit Recovery Enabled
- Course Length: 18 weeks
- Course Type: Basic
- Category:
- Science
- High School
Schools and Districts: We offer customized programs that won't break the bank. Get a quote.

Shmoop's Physics course has been granted a-g certification, which means it has met the rigorous iNACOL Standards for Quality Online Courses and will now be honored as part of the requirements for admission into the University of California system.
This course has also been certified by Quality Matters, a trusted quality assurance organization that provides course review services to certify the quality of online and blended courses.
Shmoop's Physics course has been granted a-g certification, which means it has met the rigorous iNACOL Standards for Quality Online Courses and will now be honored as part of the requirements for admission into the University of California system.
Welcome to Semester B of Physics a.k.a. Physics Part Deux a.k.a. Dude, Who Moved My Particle? a.k.a. You've Got Your Light-is-like-a-wave Theory on my Light-is-like-a-particle Theory a.k.a. Electromagnetism and Circuits Make The World Go 'Round.
As the many aliases suggest, things are changing in the course. And they really are. We're shifting from studying the motion of big stuff, to studying invisible stuff. Spooky? Maybe sometimes. Cool? Definitely always. We'll explore electromagnetism, circuitry, thermodynamics, and quantum theory in the same thoroughly explained, experiment-aided, Shmoopy way in which we explored motion, forces, and energy in the first semester of the course.
Through NGSS-friendly readings, experiments, practice problems, and activities, this semester will cover:
- heat flow and work work work.
- entropy (anarchy!) and the thermodynamics (the rule of law).
- electricity and magnetism. They might look unrelated to you now, but you'll change your tune soon enough.
- wave phenomena like refraction, diffraction, resonance, polarization, and stadium crowds.
- we'll take a peek at modern physics. Enough of that Paleolithic physics we've been doing so far—that's so 26 seconds ago.
As we continue exploring the universe through physics we'll continue learning ways in which to mystify our enemies and impress our friends, so what are we waiting for?
By the way, this is Semester B of a two-semester course. Check out Semester A if you haven't already.
Technology Requirements
Technology wise, this course requires a web browser-capable computer and a reliable internet connection. A PC too old school? A tablet will do just fine if you don't mind typing on it. Access to a scanner or digital camera, or a cellphone with a camera, or jeez, even a webcam, is a must since you'll occasionally need to upload images of diagrams.
Required Skills
Knowledge of Algebra concepts
Unit Breakdown
10 Physics—Semester B - Heat and Thermodynamics
Feeling the heat around the corner? You're not imagining it. But, no, we're not walking out on everything in 30 seconds flat—that's a different heat. Instead, in this unit we'll take our magnifying glasses and lab coats and investigate heat: heat's various types, converting between temperatures, heating curves, temperature change, work, and heat engines.
11 Physics—Semester B - Electric Fields
Ever doubted that tiny things can make big impacts in their surroundings? If so, this unit will make sure we never doubt again. That's because tiny electrons have a huge influence on their surroundings. Ever heard of electricity? Yeah, that's the work of electrons. In this unit, we'll learn everything about electricity, static or otherwise, Coulomb's Law (our newest best friend), and Gauss' Law. We'll continue exploring how systems do work and have work done on them. That being said, electricity as a concept can be a bit nebulous, so we'll ground our understanding of it by comparing it to the force we know a lot about: gravity.
12 Physics—Semester B - Magnetic Fields
Who said electricity came alone? Electricity and magnetism are the universe's best BFFs: totally inseparable and completely interdependent. And just like electricity creates fields, so does magnetism. Magnetic fields create an interesting space for moving particles since the field might change the course of those particles in very specific ways, but no worries, we'll learn how magnetic fields and charges interact with one another. Throughout the unit, we'll constantly point to real-world applications of magnetic fields and how they make our day-to-day awesome.
13 Physics—Semester B - Circuits
Ever wondered what's inside a computer or phone that allows us to do everything from sending Instagram photos to typing love letters to ourselves? The answer is simple: circuits. But circuits aren't necessarily simple. We'll discuss the parts of circuits, how to read and make circuit diagrams, how to calculate the power and capacitance of circuits, and how to use Ohm's Law and Kirchoff's Laws to determine the current and voltage in complex circuits. We'll wrap up the unit by exploring several renewable energy sources beyond wind and solar.
14 Physics—Semester B - Waves
Catch a wave and you're sitting on top of the world; catch two waves and, with the physics knowledge we're about to learn, you can have a rudimentary conversation with anyone or anything across in the universe. Cool, no?
Get ready to learn everything about waves, their types, characteristics, how they interact, how they travel, and their applications.
15 Physics—Semester B - Modern Physics
It's time to bust into the 20th century and peek into what physicists have been up to over the last century. So what have they done? Well, we'll settle the whole "is light a particle or a wave?" thing, explore the photoelectric effect, the flavor of every subatomic particle, and EM radiation. Let's just say modern physics is a doozy.
16 Physics—Semester B - Final Exam Review
We wouldn't let you walk the plank without some floaties, and we won't let you take the final exam without some review. We'll review thermodynamics, electric and magnetic fields, circuits, and modern physics, and then we'll take the final exam.
Recommended prerequisites:
Sample Lesson - Introduction
Lesson 10.02: Heat Transfer
What's invisible to the naked eye, but can cause our tongues to burn and our fingers to freeze in the snow?
Heat transfer, of course.
What regulates Earth's temperature and allows deep-fried ice cream to exist?
Yep, heat transfer again.
We've talked about heat moving from one place to another, but we haven't talked about how it moves, or transfers. In this lesson, we'll explore the slightly freaky world of heat transfer. First, we'll discuss the concept of absolute zero. (This will lead in nicely to one of our two activities: converting between temperature scales.)
After getting the low down on absolute zero, we'll learn about the three ways heat is transferred. Heat transfer is a strange world with different transfer systems. As humans, we move around using our legs, wheels, planes, dogs, amphibious creatures, etc. Heat is transferred via conduction, convection, and radiation.
Without heat transfer, Earth would be a very different place. In all likelihood, so different that life wouldn't be able to exist. For that matter, neither would the planets, the sun, solar systems or galaxies. Once we put it like that, heat transfer suddenly seems way more important than just deep frying some French fries. Fries are important, too, just maybe not as important as the existence of the universe as we know it.
Sample Lesson - Reading
Reading 10.10.02: You Give Me a Fever
We're Absolutely Certain This is Important
We know now that temperature is the measure of how fast the atoms inside something are wiggling. Higher temperatures mean faster wiggling. Lower temperatures mean less wiggling.
We also said that atoms are always wiggling, even when it's really, really cold. Well, prepare for a curveball. It turns out it's possible to get something so cold that its atoms quit wiggling altogether. At least in theory.
This uber-cold state is what is known as absolute zero, the temperature at which all molecular motion stops. As the name suggests, absolute zero is the lowest temperature that anything could ever reach. That temperature was determined to be -273 °C, i.e. really stinkin' cold.
Scientists have never been able to get anything down to absolute zero, but they've gotten really close, like within several hundred nano-kelvin.
Speaking of kelvin, where the heck did that unit come from? Let's discuss.
The problem with the standard temperature scales—Celsius and Fahrenheit—is they weren't designed to take into account the concept of absolute zero.
The Celsius scale is based on the properties of water, specifically the freezing point (0 °C) and boiling point (100 °C). That made sense for a long time, but once scientists realized there was such a thing as absolute zero, it made more sense to make absolute zero = 0, so they created a new unit, the kelvin.
Kelvin uses the same scale as Celsius, but starts at absolute zero instead of the freezing point of water. That's why we get easy conversions between the two.
- 0 K = -273 °C
- 273 K = 0 °C
- 373 K = 100 °C
To convert Celsius to kelvin, we just add 273. The formula looks like this:
Tk = Tc + 273
where Tk is the temperature in kelvin and Tc is the temperature in degrees Celsius.
The nice thing about working with kelvin is that we'll never have to use negative numbers. The downside to using kelvin is that most people are way more familiar with the other temperature scales.
Here in the USA, we're most familiar with Fahrenheit, which basically has nothing to do with kelvin. It has a different scale and a different zero point, meaning messier conversions. It's nothing crazy, though.
The easiest way to do the conversion is to first change Fahrenheit to Celsius:

where Tc is the temperature in Celsius and Tf is the temperature in degrees Fahrenheit. From there, we just add 273 to get to kelvin.
The formula looks a little wonky, but it makes sense if we think about the freezing point of water again. In Celsius, water freezes at 0°, while in Fahrenheit, water freezes at 32°. That's where the -32 comes from in the formula, to get both formulas at the same starting point. The 5/9 fraction is just the ratio between the scale, or relative size, between °C and °F.
If that's a bit much, no worries. Just remember the formula to convert between Fahrenheit and Celsius, and the formula for Celsius and kelvin and we're good to go.
Now Back to Moving Atoms
As we mentioned in the intro, heat transfer is super important. Even if we're more interested in cell phones, computers, and engineering than we are in the universe or how food gets cooked, heat transfer is vital.
Those computers, tablets, or phones we're all working on right now? They'd be fried if engineers didn't figure out how to get the processors to cool down with lightning quick heat transfer.
The cozy buildings we hide out in when it's stupidly hot or cold outside? Designed to minimize heat transfer to keep us safe from the elements.
Heat transfer comes in three varieties, the most well-known one being conduction, so we'll start there.
The Sword of Conduction
Whether we know it or not, we're all familiar with conduction. At some point in our lives we've burnt ourselves, probably rebelling against someone telling us to wait for some delicious cookies to cool. That burn happened thanks to conduction.
Conduction happens around us a bazillion times a day, whether we're eating a hot slice of pizza, freezing our fingers while playing in the snow, or opening the car door. The key to conduction is this: the objects must be in physical contact with one another, and the objects must be at different temperatures.
Let's see how this works.
Imagine we stick a cold metal sword in a hot flame (it's Saturday afternoon and we're bored). Atoms in the sword are stuck in place because the metal is in its solid state. That doesn't mean the molecules aren't moving, though. They're wiggling around constantly, just not super-fast since the sword is cold.
As the flame warms up the end of the sword, the atoms vibrate with greater amplitudes as heat transfers from the flames into the part of the sword that the flame is touching. These hot sword atoms, in turn, push on their neighbors and transfer some of the energy of their vibrational motion to those neighbors. Now, the neighbors vibrate a bit crazier and push on their own neighbors, and the neighbors of the neighbors make their own neighbors vibrate, and so on.
Eventually, the heat energy from the flame has been transported way into the metal sword by all this pushing around. If we're foolish enough to still be holding on to the metal handle of the sword, we'll be in for a sore surprise.
This is the process of conduction.

See Ya Later, Insulator
Not all materials are created equal, though. Some are good heat conductors, and others poor conductors. The poor conductors, we call them insulators. Metals are very good conductors. That's because in addition to conducting heat by their atoms pushing on other atoms, they have what are called free electrons. Free electrons are, well, free. They don't need to stick just to their own atom; they can move around between atoms and carry energy with them, conducting all along.

On the other side of the ring, plastics are wimpy heat conductors. So are ceramics, and the Styrofoam cooler sitting the garage, as well as wood and carpet. That's because these materials have no free electrons. Don't feel sad for them. These materials have their own super powers. They have the power of insulation.
While conductors transfer heat without dropping a sweat, insulators keep heat from transferring. The electrons in an insulator are tightly bound to their atoms, which prevents those electrons from flowing around carrying heat with them. Without the free electrons, very little heat is conducted. Insulators are the reason our homes stay nice and toasty in the winter. They're also how coolers keep our soda cold and thermoses keep our soup warm.
The prize for the best insulator/worst conductor goes to: a vacuum. Not the Hoover in the closet. No, not that one. In physics, a vacuum is when there is nothing, zilch, nada, just empty space. Since there are no atoms or molecules, nothing can push on anything, so no conduction can take place at all.
The more spread out atoms and molecules are, the less they're able to collide with one another, so the less heat they're able to transfer heat via conduction. Air and other gases are insulators because their atoms and molecules are more spread out than in solids.
That's not to say that air and gases can't transfer heat at all. They do, in fact, transfer heat all the time. They just have their own trick for doing so.
Convection: Going with the Flow
Now that we have conduction out of the way, we can talk about convection. It happens only in fluids. Definition alert. We call any substance that can flow a fluid. Root beer float: a fluid. Water: a fluid. Ketchup: a fluid. Air: also a fluid.
Hold it. Air is a fluid?
Yep, air is a fluid. Air flows, so it's technically a fluid. All gases are fluids. If it can flow, then it's a fluid.
Heat transfer via convection happens naturally when two parts of a fluid are at different temperatures. The hotter part of the fluid expands, which makes it less dense. Once it's less dense, that part of the fluid rises up. Meanwhile, good ol' gravity causes cooler, denser air to flow down and occupy the area where the hot air was just a moment before.
This whole process creates a circulation of air. If we have a continuous source of external heat, we'll eventually heat up all the air via convection.

A good example of convection is how Earth's atmosphere heats up. Basically, the surface of Earth warms up when rays from the Sun hit it. As the ground gets hotter, it warms the air above it. As the air becomes warmer, it rises. Then cool air swoops in to take its place and gets heated up by the hot asphalt or what have you.
Convection happens when a liquid boils, too. Without convection it would take forever to warm up water because the water could only heat via conduction from the burner. Instead convection allows the water to move around as it gets warmer and less dense and carry heat around with it.

The main difference between conduction and convection is that during conduction, only the heat moves around. During convection, some of the fluid (mass) also moves around carrying the heat with it.
A slightly different kind of convection is forced convection. That happens when a fluid is pushed around because of an outside force, like a fan or someone burping. For example, convection ovens are special ovens with fans in them. They get air molecules moving around, dancing the tango, so we don't have to wait as long for our pizza.

So Radiant
The last way heat can be transferred is through thermal radiation. This happens thanks to the wonder of electromagnetic waves. Visible light is an electromagnetic wave, and so are radio waves, microwaves, infrared waves, ultra-violet light, x-rays, and gamma rays. All these electromagnetic waves have energy, and they move at the speed of light carrying energy with them.
Everything—humans, snails, Jupiter, the Sun, a shell at the bottom of the ocean, etc.—is constantly absorbing and radiating energy (heat) by electromagnetic waves.
The hotter an object is, the more heat it radiates as electromagnetic waves. The Sun is super-hot, almost a whopping 6000 K hot. Thanks to radiation, we can bask in all that energy from the sun, keeping us nice and toasty. In fact, the radiation from the Sun is the source of energy that keeps all life on the planet going.
When the radiation from the Sun hits us, the molecules that make up our skin absorb some of that radiation in the form of heat and start to wiggle faster. This is why we can feel the heat from direct sunlight, even on cold days when the air itself is frigid.
The Sun doesn't have a patent on radiation though. Anything with a temperature emits electromagnetic waves. Here on Earth, most objects emit infrared rays, which don't have a ton of heat. Infrared is detectible, though, with devices like night vision goggles and infrared cameras.
Unlike conduction, where objects need to touch, and convection, which depends on the motion of fluid, radiation can travel through empty space or a total vacuum. That's the magic of electromagnetic waves.
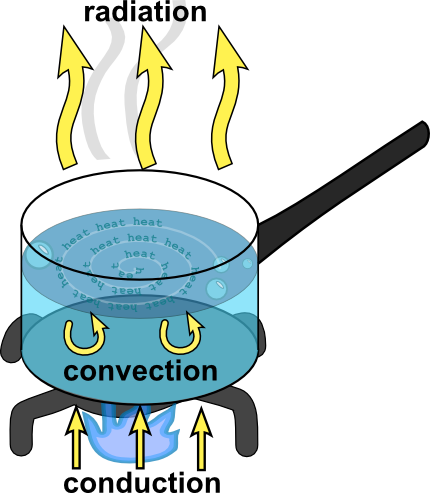
Let's review now. There are three ways heat is transferred.
- Conduction, which happens when objects are touching.
- Convection, which happens when heat is carried along with the movement of a fluid.
- Radiation, which is the transport of heat via electromagnetic waves.
Or, if you prefer a video problem recap:
And, that's all folks. Don't forget to tip the waiter.
Sample Lesson - Activity
Activity 10.02b: Hot Drawings
In the reading, we talked about hot swords and hot days, but examples with modern technology are where it's at when it comes to heat transfer. To make sure you have a handle on how heat transfer works, you're going to research a modern device that in some way utilizes heat transfer. From there, you'll draw a diagram that shows how that device is dependent on heat transfer.
Your diagram should include a drawing of the basic components of your device that are reliant on heat transfer. So for example, if you pick a car, you'd only want to draw the radiator or heater or A/C, but not the engine itself—that's a bit complicated.
We're not looking for architect-caliber drawings here, just basic drawings. Your diagram should be labeled to point out what's what. Specifically make sure to write in where conduction, convection, and radiation are happening (although not everything will have all three). If there's movement of parts or fluids, make sure to draw in arrows to show us that, too.

This diagram of a pot of water we saw from the reading is basically what we're looking for. You'll just be picking something slightly more complex. (Seriously, don't draw us a teapot, or any form of a pot or pan for that matter. Been there, done that.)
Here are some ideas you can use, or you're welcome to pick something else, as long as heat transfer plays a big role in it:
- Furnace
- Car radiator
- Air-conditioner
- Refrigerator
- Tanning bed
- Fast food heating lamps
- Heat sinks on computers
Just a heads-up, A/Cs and refrigerators are bit more complex than the other choices, so only choose them if you want a challenge. Also, we do recommend that you not use microwaves as your subject for this activity. You'd need to know stuff about microwaves that we haven't gotten to yet. Don't worry, we'll get there.
When doing research online on your technological device, make sure to use reliable sources. The website for How Stuff Works is a good place to get started. Just remember, most sources won't spell out exactly what type of heat transfer is being utilized, so you'll have to use what we learned in the reading to determine whether conduction, convection, or radiation, or some combination thereof are in play.
When you're all done with your diagram, scan or take a pic of it and upload it below.
Science Visual Presentation Rubric - 50 Points
Sample Lesson - Activity
Sample Lesson - Activity
- Credit Recovery Enabled
- Course Length: 18 weeks
- Course Type: Basic
- Category:
- Science
- High School
Schools and Districts: We offer customized programs that won't break the bank. Get a quote.
