We know that the ocean is a carbon sink and that the burning of fossil fuels is increasing the concentration of carbon dioxide in the atmosphere. Up to 50% of this excess CO2 is absorbed in the ocean. When CO2 dissolves in water, it forms a weak acid known as carbonic acid:
Reaction 6.4: CO2 (aq) + 2H2O → HCO3-(aq) + H3O+ (aq)
The increase in H+ ions released from carbonic acid shifts the reaction toward bicarbonate (HCO3-), and away from carbonate (H3O+). The end result is that less calcium carbonate is available to marine organisms.
This formation of carbonic acid can be illustrated using some cabbage juice indicator, straws, and various water samples. Add cabbage juice to each cup of water to record the starting pH. Then, using the straw, blow bubbles in each cup of water for 30-60 seconds. The color should change, indicating a change in pH. Unless you exhale different gases than everyone else, the pH will decrease. How much it decreases depends on the starting pH and the alkalinity of the water.
On a much larger scale, this is what is occurring in the ocean. This process is known as ocean acidification. The current pH of the ocean hovers around 8.1, but the projected pH for the end of the century is 7.7. This drop in pH will directly affect calcifiers, organisms that use calcium carbonate to build their shells. Examples of calcifiers include marine snails, crabs, sea urchins, lobsters, and coral. As pH levels plummet, the amount of calcium carbonate available to organism also plummets.
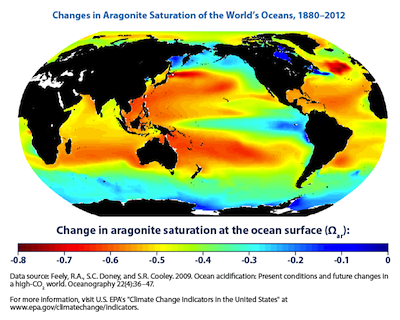
Some types of calcifiers use aragonite to build their shells. The diagram below shows the change in aragonite saturation in the oceans from 1880 to 2012. In areas with large decreases in aragonite saturation (indicated in red and orange), calcifiers that depend on aragonite will likely decrease their calcification rates. The effects could range from thinner shells and skeletons, to the complete dissolution of shells. Of course, other factors like rising temperatures also affect calcification rates. (Source)

It is likely that different types of calcifiers will respond differently to ocean acidification. In experiments, crabs and lobsters have built heavier thicker shells when exposed to lower pH levels. Sea urchins and pteropods tend to build thinner shells as pH levels plummet.(Source)
In addition to affecting calcification rates, increasing levels of carbon dioxide also affect reproductive success by either slowing down sperm or causing problems in larval development. There have been recent reports of urchins that have already adapted to lower pH levels. Genes controlling ion transport in purple sea urchins exposed to lower pH levels show more changes than those same genes in purple sea urchins not exposed to lower pH levels27.

New research indicates that purple sea urchins might be able to keep up with ocean acidification. Urchins exposed in the lab to lower pH levels seemed to be able to evolve quickly by mutations in genes controlling ion movement into and out of the cell. Scientists think the sea urchin's unusual degree of genetic variability might be what allows for this rapid evolution. (Source)
Photosynthetic organisms, like algae and seagrass, might benefit from increasing levels of carbon dioxide in the ocean. From biology, we know that carbon dioxide is a reactant in photosynthesis. More carbon dioxide, then, means greater rates of photosynthesis. This is good news for photosynthesizers and anything that consumes them.