Lab Tips in Types of Bonds and Orbitals
Ready to jump into some practical stuff? Well get your lab coat, gloves, and protective eye goggles on. It's time to take a journey into the lab. Cue ominous and haunting music.
It's important to make sure you always follow the directions very carefully in your lab book and manual and never arbitrarily mix substances that you have no idea about.
Now onto the fun stuff—this virtual lab is going to first describe the different properties of covalently bonded compounds and ionically bonded compounds. Then, we will join in on an actual lab video to see how compounds behave differently depending on whether they are made up of covalent bonds or ionic bonds.
First let's recap on the basic definitions of covalent bonds and ionic bonds.
Metals + Nonmetals → Ionic Bonds
Nonmetal + Nonmetal → Covalent Bonds
Now let's get into some general properties of each of these bonds (remember that these are general properties and that there are always special exceptions to every rule).
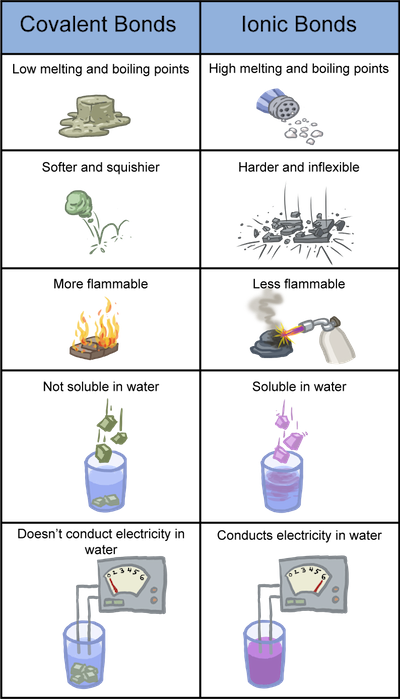
Now that we know the general properties of these bonds, we can watch an online experiment where we measure these properties in compounds to help us identify which compounds contain either covalent or ionic bonds. Some examples are salt and sugar. This video tests the melting point, solubility, and conductivity of various compounds to determine whether the compounds contain either covalent or ionic bonds. Watch and be amazed.
Use the table below to indicate the different properties observed in the experiment.
| Type of Compound | Melting Point | Solubility in Water | Conductivity |
| Sucrose C12H22O11 | |||
| Sodium Chloride NaCl | |||
| Oxalic Acid C2H2O4 | |||
| Cobalt (II) Sulfate CoSO4 | |||
| Nickel (II) Chloride NiCl2 | |||
| Starch C6H10O5 |
For more details on why these properties exist for covalent and ionic bonds please refer to this website.