The Organic Chemistry Behind Medicines
Our bodies contain thousands of organic compounds. Proteins, carbohydrates, nucleotides, and lipids are some examples that we learned about in this chapter.
Pharmaceuticals are also organic compounds. And, while we are constantly bombarded with pharmaceutical advertisements on both TV and in popular magazines, we probably don't pay close attention to these, and we definitely don't pay attention to the accompanying fine print that is full of confusing medical terms.
However, upon a closer look, we'd be surprised at how much we are able to understand from the organic chemistry we've learned in this chapter. Remember, both doctors and scientists started where we are right now…introductory organic chemistry.
Let's take a look at a few common pharmaceuticals.
1. Lipitor6
One of the best selling drugs in the United States is Lipitor. High cholesterol is a common problem, with 1 in 5 adults having been diagnosed with high cholesterol. What exactly is cholesterol?
Cholesterol is an organic molecule involved in building cell membranes. In the diagram of cholesterol below, we can see several functional groups that we discussed previously in this chapter, including a hydroxyl group, a hydrocarbon chain, several hydrocarbon rings, and even a double bond:

The hydroxyl group allows for cholesterol to interact with other organic molecules. Specifically, cholesterol interacts with the fatty acids, another type of molecule we discussed in this chapter.
If your cholesterol levels become too high, cholesterol plaques stick to the walls of arteries and inhibit blood flow. This is similar to when a large chunk of ice cream gets stuck in your milkshake straw, but much more serious (incidentally, too many milkshakes might be why our cholesterol levels are so high in the first place, but we digress).

In comes Lipitor, the cholesterol hero. The molecular structure of Lipitor also contains functional groups we know. Hydroxyl groups, benzene rings, carboxylic acids, amines, and ketones…the gang's all here.

Together, Lipitor's functional groups interact with cholesterol's functional groups, resulting in the breakdown of cholesterol and making the world a better place.
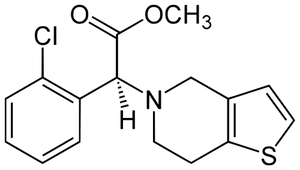
2. Plavix
Blood clots result from platelets aggregating together and forming a type of plug in blood vessels.
Blood clots don't always form where and when we'd like them too. When we need to remove unwanted blood clots, who ya gonna' call? Clot busters!
Plavix is a drug used to inhibit blood clots, or bust them, if you will. The molecular structure of Plavix contains a benzene ring, a halogen, an amide, and an ester. It also contains an inorganic sulfur atom, a functional group covered in a previous chapter.
3. Singulair7,8
Does thinking about pollen make you want to sneeze? If so, you are not alone.
Singulair is used to treat seasonal allergies and some types of asthma. It contains a whole slew of functional groups; benzene rings, hydroxyl groups, carboxylic acids, amines, double bonds and a halogen are all included. Luckily we don't have to learn how to name this one.

Singulair works to block allergic reactions in our lungs before they can happen. That's right, all those functional groups you see in this diagram are just a bunch of bodyguards, working to keep your lungs protected.