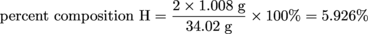Percent Composition
Another common type of stoichiometry problem on exams and quizzes involves calculating percent composition, which is the percent by mass of each element in a compound.
Suppose a pharmaceutical company needs to verify the purity of a new drug they have made. They first would figure out what the percent composition for each element is supposed to be. Then they would compare that result to the percent composition obtained experimentally. If the numbers don't match, that means the compound is not pure and the chemists have some work to do.
How would we go about calculating this number? We've already learned that the formula of a compound tells us the number of atoms of each element in a single molecule. For example, methane has the formula CH4. In English, that's one carbon atom and four hydrogen atoms.
The percent composition is obtained by dividing the mass of each individual element in 1 mole of the compound by the total molar mass of the compound. That gives us the fractional composition. To get the percent composition we just need to follow up with a quick multiplication by 100. For all those math lovers, let's look at the definition expressed in equation form:

where n is the number of moles of the element in 1 mole of the compound.
Back to the CH4 example: in one mole of methane, there are 4 moles of hydrogen and 1 mole of carbon. We can also say that for every four moles of hydrogen there is 1 mole of carbon. Po-TAH-to, po-TAH-to. We already calculated the molar mass of CH4 to be 16.042 g. Our handy-dandy periodic table informed us that the molar mass of carbon is 12.01 g and the molar mass of hydrogen is 1.008 g.


How do we check our percent composition answers? Add up all of the percent composition values. If your answer is not 100%, then Houston, we have a problem. Keep in mind that, due to rounding, the values might not always be exactly 100%; they'll be very close, though.
Speaking of problems, let's try another to make sure we've got the hang of this.
First of all, let's calculate the molar mass of H2O2 knowing that the molar masses of H and O are 1.008 g and 16.00 g, respectively.
molar mass H2O2 = 2(atomic mass of H) + 2(atomic mass of O)
molar mass H2O2 = 2(1.008 g) + 2(16.00 g) = 34.02 g
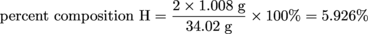

Suppose a pharmaceutical company needs to verify the purity of a new drug they have made. They first would figure out what the percent composition for each element is supposed to be. Then they would compare that result to the percent composition obtained experimentally. If the numbers don't match, that means the compound is not pure and the chemists have some work to do.

How would we go about calculating this number? We've already learned that the formula of a compound tells us the number of atoms of each element in a single molecule. For example, methane has the formula CH4. In English, that's one carbon atom and four hydrogen atoms.
The percent composition is obtained by dividing the mass of each individual element in 1 mole of the compound by the total molar mass of the compound. That gives us the fractional composition. To get the percent composition we just need to follow up with a quick multiplication by 100. For all those math lovers, let's look at the definition expressed in equation form:

where n is the number of moles of the element in 1 mole of the compound.
Back to the CH4 example: in one mole of methane, there are 4 moles of hydrogen and 1 mole of carbon. We can also say that for every four moles of hydrogen there is 1 mole of carbon. Po-TAH-to, po-TAH-to. We already calculated the molar mass of CH4 to be 16.042 g. Our handy-dandy periodic table informed us that the molar mass of carbon is 12.01 g and the molar mass of hydrogen is 1.008 g.
Sample Problem
Calculate the percent composition of carbon and hydrogen in methane.

How do we check our percent composition answers? Add up all of the percent composition values. If your answer is not 100%, then Houston, we have a problem. Keep in mind that, due to rounding, the values might not always be exactly 100%; they'll be very close, though.
Speaking of problems, let's try another to make sure we've got the hang of this.
Sample Problem
What is the percent composition of hydrogen and oxygen in hydrogen peroxide, H2O2?First of all, let's calculate the molar mass of H2O2 knowing that the molar masses of H and O are 1.008 g and 16.00 g, respectively.
molar mass H2O2 = 2(atomic mass of H) + 2(atomic mass of O)
molar mass H2O2 = 2(1.008 g) + 2(16.00 g) = 34.02 g