ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Laws of Thermodynamics Videos 15 videos
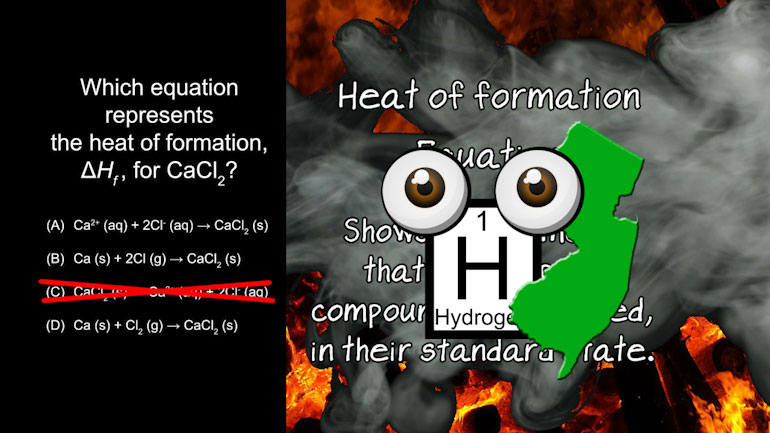
AP® Chemistry: Laws of Thermodynamics Drill 1, Problem 1. Which equation represents the heat of formation for Calcium Chloride?
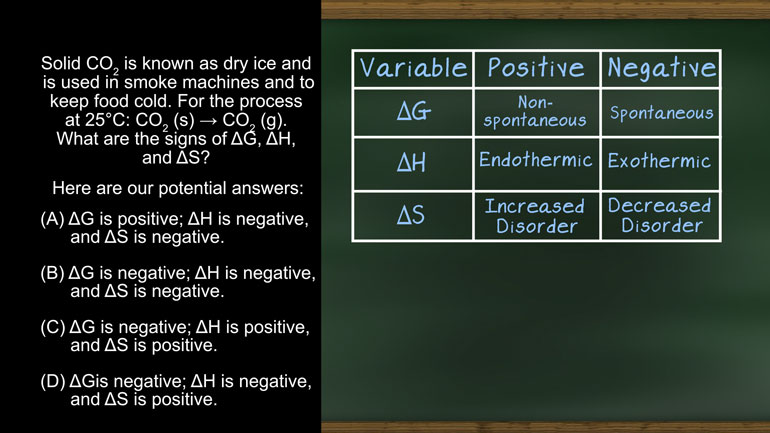
AP Chemistry 2.2 Laws of Thermodynamics. What is the ΔG and the spontaneity of the reacton?
AP Chemistry 2.2 Laws of Thermodynamics 30 Views
Share It!
Description:
AP Chemistry 2.2 Laws of Thermodynamics. What is the ΔG and the spontaneity of the reacton?
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by spontaneity.
- 00:07
Otherwise known as why we're not going to finish this Shmoop du jour. [Shmoop employee at a desk and disappears]
- 00:10
We're going to Mexico, instead.
- 00:11
Vamos! [Car driving to Mexico]
- 00:12
Okay…maybe our spontaneous trip can wait.
Full Transcript
- 00:16
Here's our question.
- 00:17
Consider the following reaction…right here…
- 00:19
Done checking it out?
- 00:21
Okay, moving right along…
- 00:23
The enthalpy of formation for the reaction is -92.0 kJ, and the change in
- 00:29
entropy is -55.4 J/K at 30 °C. What is the DeltaG of the reaction, and
- 00:37
what is the spontaneity of the reaction, respectively?
- 00:40
And here are your potential answers. [Potential answers to the reaction]
- 00:43
So we’re given a few different pieces of information in this problem. [Puzzle pieces separated]
- 00:48
We’ve got values for the enthalpy or heat of formation,..
- 00:51
…the entropy change,..
- 00:52
…and the temperature.
- 00:53
And we’re asked for the ?G, or the change in Gibbs free energy.
- 00:57
And no, by free energy, we don’t mean a blow-out sale on Red Bull. [Girl shopping for packs of Red Bull]
- 01:02
The Gibbs free energy tells us about the spontaneity of a reaction.
- 01:06
We need to know the value, and specifically the sign, of DeltaG.
- 01:11
We define DeltaG using the Gibbs free energy equation, DeltaG=DeltaH-T DeltaS
- 01:16
Anybody else craving alphabet soup? [Dinner lady serves boy alphabet soup]
- 01:20
We have values for all of the variables on the right hand side of this expression.
- 01:24
But before we plug them in, we have to consider their units.
- 01:27
There’s a few sneaky little guys that we need to convert before we plug them in, plug [Person plugs B into a wall socket]
- 01:31
them in.
- 01:33
The answer needs to be in kilojoules, so first convert DeltaS to kilojoules by multiplying
- 01:37
by the number of kilojoules in a joule.
- 01:40
And let’s not forget about temperature — DeltaS includes a kelvin unit and our given
- 01:45
temperature is in Celsius.
- 01:47
The kelvin scale is an absolute scale––it has a value of zero at absolute zero––so [Woman stood on a weighing scale]
- 01:53
we typically choose to use Kelvin in our equations.
- 01:55
Are we sure that’s better?
- 01:58
Absolutely.
- 01:59
Converting our temperature units, we find that our temperature is 303.15 Kelvin.
- 02:03
Now we just have to do the math…
- 02:04
Chugga-chugga-chugga, bing-bang-boom, and…
- 02:06
DeltaG= -75.2 kJ [Answer for the reaction]
- 02:12
Looking again at our choices, we see that choice D is what we want.
- 02:15
But we’re not done yet!
- 02:17
Is this reaction actually spontaneous? [Couple talking beside a stream]
- 02:20
Spontaneity depends on the sign of DeltaG
- 02:22
A negative DeltaG, a change in Gibbs free energy means energy is released and a reaction is
- 02:28
spontaneous, and a positive DeltaG means energy is added and
- 02:32
the reaction is not spontaneous.
- 02:35
So the negative DeltaG we calculated does correspond to a spontaneous reaction.
- 02:39
High five. [Boy holding out a hand for a high five]
- 02:40
Seriously.
- 02:41
Slap your screen. [Person slaps boys hand for high five]
- 02:42
Don't leave us hanging…
- 02:43
Now come on!
- 02:44
Mexico awaits!
- 02:45
…No?
- 02:46
You guys have responsibilities?
- 02:47
Drat.
- 02:48
Spontaneity looked awfully good in the movies… [Car spontaneously drives to Mexico]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?