ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Structure and Arrangement of Atoms Videos 9 videos
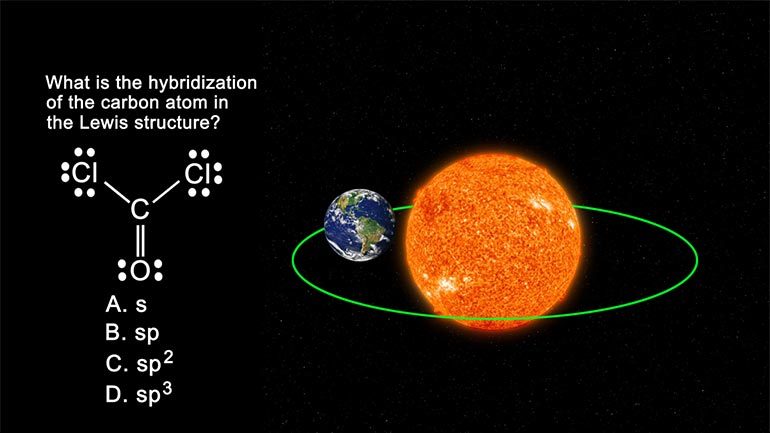
AP Chemistry: Structure of Atoms Drill 1, Problem 5. What is the hybridization of the carbon atom in the Lewis structure?
AP Chemistry 2.5 Structure and Arrangement of Atoms. Under which of the following conditions would a real gas behave more like an ideal gas?
AP Chemistry 3.5 Structure and Arrangement of Atoms. Which of the following is true regarding solids?
AP Chemistry 3.3 Structure and Arrangement of Atoms 15 Views
Share It!
Description:
AP Chemistry 3.3 Structure and Arrangement of Atoms. What is the direct conversion from a solid to a gas called?
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by states of matter.
- 00:08
Chemistry has three, which is perfectly respectable. [Solid, liquid and a gas]
- 00:10
America’s just showing off.
- 00:13
Here’s our question:
- 00:15
Solid carbon dioxide is sometimes used to refrigerate foods and to make the fog that
Full Transcript
- 00:19
emits from fog machines because carbon dioxide is a solid at low temperature.
- 00:25
When exposed to air, carbon dioxide immediately converts to a gas.
- 00:29
What is the direct conversion from a solid to a gas called?
- 00:33
And here are our potential answers...
- 00:38
This question is fairly straightforward, but just for fun, let’s look at all the different [Road sign for route to the answer]
- 00:42
phase transitions you might get thrown at you on a test.
- 00:45
Hopefully not literally. [Teacher throwing balled up paper at a student]
- 00:47
Matter exists in one of three states: solid, liquid, or gas.
- 00:52
The temperature of the matter and pressure on the matter will determine what state the
- 00:56
matter is in.
- 00:58
Matter can transition between states if the pressure or temperature of the matter changes, [Liquid pouring out of a soda bottle]
- 01:02
or if they’re standing in that one cool place where Utah, New Mexico, Arizona, and
- 01:06
Colorado meet.
- 01:07
The most common transitions we observe in daily life are solid to liquid and liquid
- 01:12
to gas, because these are the transitions that water undergoes at normal temperatures [Man sitting on a sofa with a water droplet]
- 01:17
and pressures.
- 01:18
If these are not the transitions you observe most frequently, you may be a life form from [People standing in a field and alien appears from a spacecraft]
- 01:23
another planet in which case please contact NASA so they can study you––no dissections
- 01:29
we promise.
- 01:30
However, all matter can undergo a transition between any two phases of matter if the temperature [Girl throwing darts at a board]
- 01:35
and pressure are in the right spot.
- 01:37
The question is asking us about a transition from a solid to a gas, which isn’t common
- 01:42
in water on earth but is the norm for carbon dioxide. [A river streaming with water]
- 01:46
And because scientists like graphs...really, here’s a graph showing how much we like
- 01:50
graphs compared to other people….there’s a graphical way to represent phase transitions. [People studying a graph]
- 01:56
This helps us see how transitions between any states of matter can occur, while having
- 02:00
all the added fun of being a graph!
- 02:05
Creatively called a “phase transition diagram,”––what? [Boy with a phase transition diagram]
- 02:10
We like graphs, not thinking of creative names for them–– this plot shows the state of
- 02:14
matter for CO2, the molecule in the question.
- 02:18
Each line between states on the graph represents a point where if the temperature or pressure
- 02:23
changes, the carbon dioxide will go from one state to another.
- 02:28
This gives us a total of six ways that matter can change states, because each change is
- 02:33
reversible.
- 02:34
Let’s take a look at what each change is called.
- 02:37
Going from solid to liquid is named “Thomas,” but goes by “Tom” for short. [Ice cubes melt into a puddle of water]
- 02:41
...Okay, it’s actually called melting, and going from liquid to solid is freezing.
- 02:47
Going from liquid to gas is boiling and going from gas to liquid is condensing [Person pours boiling water into a pan]
- 02:52
And going from solid to gas is sublimation and going from gas to solid is deposition
- 03:00
The question was asking what the transition from a solid to a gas is called, so the answer
- 03:05
is A. Sublimation. [Answer A circled green]
- 03:07
Answer B, melting is solid to liquid and answer D, boiling is liquid to gas.
- 03:12
And answer D, decomposition isn’t even a transition between states of matter, so we
- 03:16
can toss that one right into the compost bin. [Man throws decomposition into a compost bin]
- 03:19
We found the right answer and eliminated all the others, so our chemistry skills must be
- 03:23
sublimation!
- 03:25
...Whoops. [Chemistry skills explode]
- 03:26
We meant sublime.
- 03:27
Now we’ll never get those skills back...
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?