Limiting Reagents
Our Reagents are Limitless
We almost have enough information to solve any sort of stoichiometry problem that our chemistry teachers throw at us. There is one very important concept that we've yet to cover…and it's a biggie. Let's revisit a reaction equation that we've already seen:N2 + 3H2 → 2NH3
Of course, we can't forget about our ever-important conversion factors:

Now that our memories are all refreshed, let's consider the following reaction. How many grams of NH3 are produced when 1 gram of N2 is reacted with 20 g H2? This seems similar to reactions we've seen before, right? What's the big deal? We just have to convert the gram quantity given to moles, use our handy-dandy conversion factor, and then reconvert to grams of product. Are we missing something? Let's solve the problem as we normally would and see what happens.
grams of H2 → moles of H2 → moles of NH3→ grams of NH3
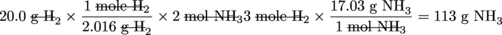
This is exactly how we solved the problem before. Trust us, we double-checked. The only difference between this problem and the problem we solved before is the fact we are now given exact quantities of both reactants (H2 and N2) as opposed to just H2 before. Let's re-do this problem starting with the N2 instead of the H2.
grams of N2 → moles of N2 → moles of NH3→ grams of NH3

Even though 20.0 g H2 can technically produce 113 g of NH¬3 we only have enough N2 to produce 12.2 g NH3.
When a chemist carries out a chemical reaction, the reactants are usually not present in the exact same quantities. The goal of any chemical reaction is to produce the maximum quantity of product based on the given starting materials. It's actually quite common to have one reactant in huge excess. That means when the other reactant is used up, the maximum amount of product is achieved and the reaction stops. Done. Consequently, some reactant will be left over at the end of the reaction.
The reactant used up first in a reaction is called the limiting reagent. When this reactant is used up, no more product can be formed. Excess reagents are the reactants present in quantities greater than necessary to react with all of the limiting reagent.
The concept of the limiting reagent is analogous to the relationship between spaghetti and meatballs. We could go to the store and buy enough ingredients to make 1,000,000 meatballs, but if the store only has 2 boxes of spaghetti to buy we're out of luck. The number of boxes of spaghetti limits the amount of plates of spaghetti and meatballs we can assemble even though there is an excess of meatballs. Steve Martin also has a similar problem purchasing hotdogs and buns.
Let's try out a simple example to make sure you're buying what we're selling. Consider the following reaction:
CO + 2H2→ CH3OH
Suppose we initially have 4 moles of CO and 6 moles of H2. One way we can quickly (and easily) determine which of the two reactants is the limiting reagent is to calculate the number of moles of the product we can achieve based on the initial quantities of CO and H2 given.
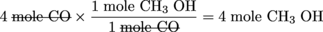
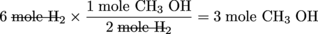
Because H2 results in the smallest amount of CH3OH formation, it must be the limiting reagent. CO is the excess reagent. Determining which of the starting materials is the limiting reagent is one of keys to solving stoichiometry calculations correctly.