Reaction Yields
We've almost reached the end of our stoichiometric journey. Almost brings a tear to our eye. Almost.
We learned that the amount of limiting reagent present at the start of a reaction determines the yield. To be more specific, this yield is called the theoretical yield. It's the amount of product that would result if the entire limiting reagent reacted. In a perfect world with perfect chemists and perfectly aligned stars, all reactions would go…perfectly. In a perfect world, we would always achieve the theoretical yield.
Unfortunately, we don't live in a perfect world. Reactions don't always go the way they're supposed to. Sometimes reactions stop before a limiting reagent is used up. Sometimes side reactions occur. Sometimes zombies come and eat our brains out before we can finish the experiment. It could happen. The theoretical yield is then the maximum amount of product that can be formed in a reaction predicted by the balanced chemical equation.
In our non-perfect world, reactions give an actual yield, or the amount of product that is actually obtained from a reaction. This amount is always smaller than the theoretical yield. There are also those few rare occurrences when the theoretical yield and the actual yield are identical, like two peas in a pod.
We want to know how well our reaction did—we're scientists, after all. Sometimes we want to compare our results to others or help determine if the reaction can be improved. There are also those times we just want to pat ourselves on the back for getting that 98% yield. To determine how efficient (or good) a given reaction is, chemists often calculate the percent yield, which describes the proportion of the actual yield to the theoretical yield. It is calculated like this:
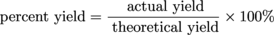
Scientists of all types strive to get the biggest percent yield of them all! Bigger is better… or so we're told! This is kind of like grades. The closer to 100% you are, the better.
N2 + 3H2 → 2NH3
If 5 g N2 is reacted with 10 g H2, what is the theoretical yield of NH3 (in grams)? If 4.0 grams is actually obtained, what is our percent yield?
Hint: Remember to first determine the limiting reagent.


Okay so N2 is our limiting reagent, and 6.08 g is our actual yield.

We learned that the amount of limiting reagent present at the start of a reaction determines the yield. To be more specific, this yield is called the theoretical yield. It's the amount of product that would result if the entire limiting reagent reacted. In a perfect world with perfect chemists and perfectly aligned stars, all reactions would go…perfectly. In a perfect world, we would always achieve the theoretical yield.
Unfortunately, we don't live in a perfect world. Reactions don't always go the way they're supposed to. Sometimes reactions stop before a limiting reagent is used up. Sometimes side reactions occur. Sometimes zombies come and eat our brains out before we can finish the experiment. It could happen. The theoretical yield is then the maximum amount of product that can be formed in a reaction predicted by the balanced chemical equation.
In our non-perfect world, reactions give an actual yield, or the amount of product that is actually obtained from a reaction. This amount is always smaller than the theoretical yield. There are also those few rare occurrences when the theoretical yield and the actual yield are identical, like two peas in a pod.
We want to know how well our reaction did—we're scientists, after all. Sometimes we want to compare our results to others or help determine if the reaction can be improved. There are also those times we just want to pat ourselves on the back for getting that 98% yield. To determine how efficient (or good) a given reaction is, chemists often calculate the percent yield, which describes the proportion of the actual yield to the theoretical yield. It is calculated like this:
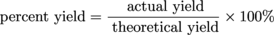
Scientists of all types strive to get the biggest percent yield of them all! Bigger is better… or so we're told! This is kind of like grades. The closer to 100% you are, the better.
Sample Problem
Let's go back to our favorite example.N2 + 3H2 → 2NH3
If 5 g N2 is reacted with 10 g H2, what is the theoretical yield of NH3 (in grams)? If 4.0 grams is actually obtained, what is our percent yield?
Hint: Remember to first determine the limiting reagent.


Okay so N2 is our limiting reagent, and 6.08 g is our actual yield.
