Combustion
Combustion reactions are an important class of chemical reactions. These reactions are vital to our everyday lives. How often do you drive around in a gas-fueled car? Or cooked a grilled cheese on a gas stove? How does your family stay warm in the winter? Perhaps a gas furnace does the trick? The combustion of fuels (wood, fossil fuels, peat, etc.) have heated and lit our homes and cooked our food for thousands of years. Our lives would be completely different if combustion reactions did not exist. Can you guess another added bonus of these gassy reactions? They provide tons of stoichiometry and balancing equations practice. Score.
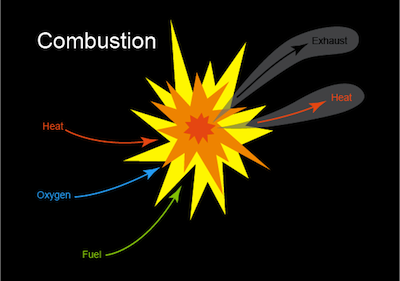
A combustion reaction takes place when fuel and oxygen react, producing heat or heat and light. The most recognizable form of a combustion reaction is a flame such as a burning candle or a nice toasty campfire. Who brought the s'mores?
Combustion usually occurs when a hydrocarbon (meaning a compound composed of carbon and hydrogen) reacts with oxygen to produce carbon dioxide and water. These reactions are also highly exothermic which means they release energy often in the form of heat. That's why combustion is a great way to heat a house or run a car engine.
hydrocarbon + oxygen → carbon dioxide + water
CH4 + O2 → CO2 + H2O
But wait—the combustion reaction is not balanced. We have more oxygen atoms and fewer hydrogen atoms on the right hand side of the equation. How could we commit such a stoichiometric crime? Let's solve our conundrum by using the atom inventory technique.
| Element | Before | After |
| C | 1 | 1 |
| H | 4 | 2 |
| O | 2 | 3 |
It looks like we need one additional water molecule on the right hand side to even out our hydrogens before and after.
CH4 + O2 → CO2 + 2H2O
| Element | Before | After |
| C | 1 | 1 |
| H | 4 | 4 |
| O | 2 | 4 |
To balance the last element (oxygen) we just need one additional O2 molecule on the left hand side.
CH4 + 2O2 → CO2 + 2H2O
| Element | Before | After |
| C | 1 | 1 |
| H | 4 | 4 |
| O | 4 | 4 |
That wasn't so hard, was it? Try balancing these two additional combustion equations on your own:
C10H8 + O2→ CO2 + H2O
C2H6 + O2 → CO2 + H2O
Answer
C10H8 + 12O2→ 10CO2 + 4H2O
2C2H6 + 7O2 → 4CO2 + 6H2O