Arrhenius Equation
It's Not as Dirty as it Sounds
Svante Arrhenius…putting the k in kinetics for 120 years. Svante Arrhenius, which roughly translates to "Savant Are a Genius," studied chemical kinetics for lots and lots of reactions. He found that the rate constant k and temperature are related mathematically. He even found an equation for it, which he named after himself. How modest.k =Ae-Ea/RT
- k = Rate constant
- A = Frequency Factor
- Ea = Activation Energy
- R = Gas constant = 8.31 J/mol K
- T = Temperature (Kelvin)
Logarithmic functions are the yin to exponential function's yang, so to speak. Logs (and their buddies natural logs) let us plot an exponential function as a line, and we all know how much chemists loves a straight line. This is the main reason that chemists use log or ln plots. By taking the log of both sides of the equation above, it transforms, Optimus Prime style, into a much easier to use form.
In other words, start with k =Ae-Ea/RT and take the log of both sides to get the following.
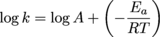
Fancy, huh?
The constants A, R, and Ea are considered to be independent of temperature. The variables k and T can change. As T increases, k increases. We can do a little mathematical gymnastics (mathnastics?) and get an equation that looks like the following. We've spelled out what pops out when we rearrange.

Looks familiar, doesn't it? It's our good ole formula for a line. As such, if we do up a quick Arrhenius plot (log k vs. 1/T), the following information can be calculated.
1. The frequency factor, A. The y-intercept is log A. If we take 10(log A), then A just drops out into our lap.
2. The activation energy, Ea. The slope of the line is
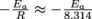 . Multiplying the slope by -8.314 gives the activation energy (in Joules/mole).
. Multiplying the slope by -8.314 gives the activation energy (in Joules/mole).