Collision and Transition State Theory
Tug of Chemical War
Have you ever wondered how reactions occur anyway? We've already learned that reactions are all about the making and breaking of bonds and the movement of electrons, but why does all of this happen?Sometimes electrons get shared, and sometimes electrons get passed along from one atom to another like a bad penny. The sharing and giving of electrons wouldn't occur if atoms didn't bounce off of each other like angry dudes in a mosh pit. This is the heart of what is called collision theory.
Collision theory basically states that reactants need to do two things for a reaction to occur.
- They need to slam into one another with sufficient force
- They also need to be at the correct angle
Using these factors, chemists have found that collision theory can be expressed mathematically. The rate constant k = Z × f × l. Just to be clear, the rate constant, k, is not the same as the rate of the reaction. Rather, the rate constant is used to calculate the rate of the reaction.
One of the things that collision theory helps to explain is why increasing temperatures result in an increase in reaction rates. Collision frequency increases with increasing temperature because atoms and molecules move more rapidly at higher temperatures. Temperature also increases the energy of collisions, which increases the value of f. Essentially, increasing temperature is a two-for-one deal for increasing the rate constant. No coupons necessary.
Now's a Good Time for a Transition
Transition-state theory goes hand-in-hand with collision theory. Transition-state theory states that a reaction follows a distinct reaction path that involves bonds being formed and being broken simultaneously. It's basically like watching a reaction in super-slow-motion, where atoms or molecules move and change position. Check out this video if you're still confused.Bond breaking and formation require energy, which is the reason that reactions have an activation energy to overcome. Remember, activation energy is the minimum amount of energy needed for a reaction to occur.
Another important concept in transition-state theory is the idea of an activated complex. Basically, the activated complex is a weird hybrid thing that is formed which is neither reactants nor products. As two reactants come closer and closer together, the atoms in each molecule start to move in response. Bonds between the reactants may start to break and new product bonds may start to form.
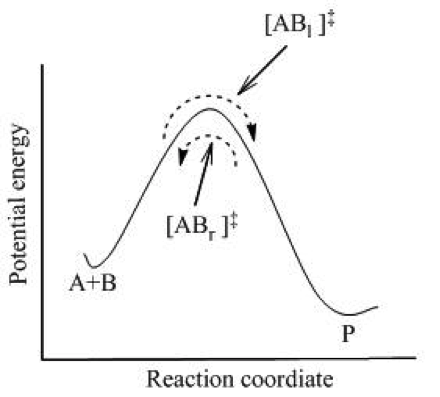
Activated complexes are highly unstable and are not observed because they are unstable. The activated complex is the point in a reaction where its potential energy is highest. Potential energy sounds scary, but just think about throwing a ball into the air. The potential energy is highest when the ball reaches its highest point. It has the most potential to do some serious damage…get it?
